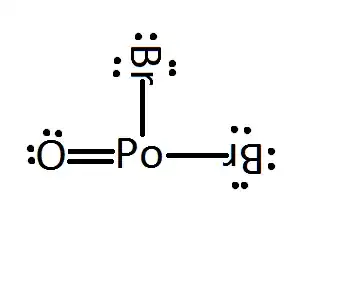For formulas that can produce only one isomer or "connective pattern" and using the typical Lewis structure bonding for the elements, start by separating the atoms into those that only make 1 bond and those that make more than one bond. In this case: 
Elements that make more than one bond have to be bonded together with at least one bond, so in this case take the Po and O and make one bond between them while leaving the other open bonding sites alone like this:
Now you have to place the monovalent elements on the multivalent elements. You could do this: 
but you have used up all of your atoms, and Polonium still needs to make 2 bonds, and it can't do it by itself, so you can see that IF you put them all in a row, you would get stuck with an atom that has to make 2 more bonds but has nothing to bond to. Instead, we need to move both of the Brs to the Po like this:

Then since Po and O each need to make one additional bond, they can form a second bond between them like this:
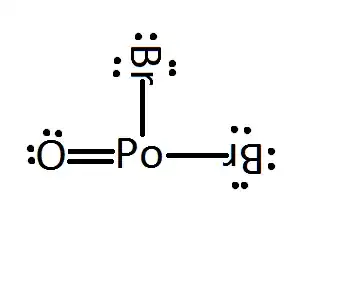
You can turn around the Br symbols and also make the bonding symmetrical if you want to: 
(Get in the habit of showing your unbonded electron pairs at least for atoms that make more than 1 bond like Oxygen and Nitrogen)