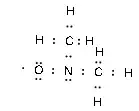
I've been taking an chemistry class online (for fun only) and one of the questions in the homework threw me a bit. Here's the wording:
"Draw the Lewis structure for a nitrogen atom attached to. two methyl groups ($\ce{CH3}$) and one oxygen atom."
OK, so I know that $\ce{NO}$ is a free radical due to the odd combined valence, i.e., the octet rule isn't satisfied. Will that carry over when the $\ce{NO}$ bonds with the two methyl groups?
Here's the sketch of what I did:

Lastly, what's the name of this compound? This is not part of the HW question; I'm just curious.