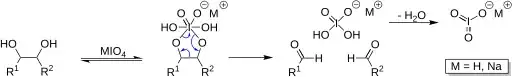
Recently, I was reading Clayden's Organic Chemistry (2nd ed.) and I chanced upon this diagram on p. 443 (as shown below) showing an octavalent iodine in the periodate ester. I found this rather puzzling as I thought that the maximum possible valency of iodine was seven. I am well aware that bonding schemes such as the 3-centre 4-electron bonding scheme allow central atoms to make more than the number we would expect the atom to make conventionally. However, this is the first time I am seeing octavalent iodine and thus, I would just like to confirm if it is in fact, possible for iodine to be octavalent.
Reference
Clayden, J., Greeves, N., & Warren, S. (2012). Organic Chemistry (2nd ed.). New York : Oxford University Press Inc.