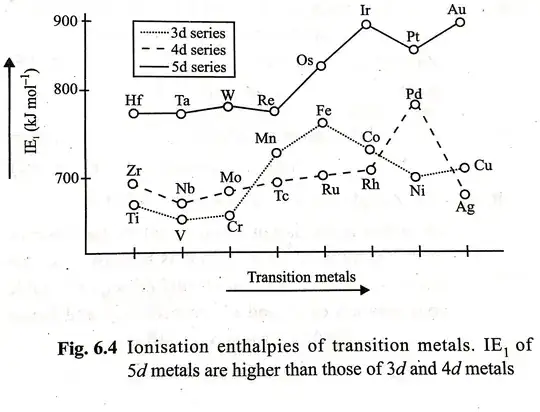
The d block elements display several exceptional behaviours, and one of them is the irregular trend in ionization enthalpy of 3d elements.
The reason given by my textbook is very confusing to me so I'm unable to grasp it. I would appreciate it if someone could explain this statement to me in a simple way.
The irregular trend in the first ionisation enthalpy of the $\ce{3d}$ metals, can be accounted for by considering that the removal of one electron alters the relative energies of $\ce{4s}$ and $\ce{3d}$ orbitals. So the unipositive ions have $\ce{d^n}$ configurations with no $\ce{4s}$ electrons. There is thus, a reorganisation energy accompanying ionisation with some gains in exchange energy as the number of electrons increases and from the transference of s electrons into d orbitals
Text source: NCERT (d & f block elements/ page 215)
Image source: Inorganic Chemistry Cengage Part-2 ( page 6.13)