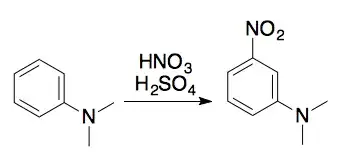
The nitration of N,N-dimethylaniline with $\ce{H2SO4}$ and $\ce{HNO3}$ gives mainly the meta product, even though $\ce{-NMe2}$ is an ortho,para-directing group. Why is this so?
- 71,033
- 11
- 239
- 410
- 2,322
- 1
- 21
- 25
-
1https://en.wikipedia.org/wiki/Tetryl - reaction can proceed in other way – Mithoron Jul 30 '15 at 22:55
-
The best way around this issue, from a reagent/yield point of view, is to use acetic anhydride to N-acetylate the primary amine. This gives acetanilide, the acetyl derivative of aniline. – Aug 30 '15 at 00:13
-
Essentially a dupe: http://chemistry.stackexchange.com/questions/31644/how-is-anilinium-ion-meta-directing-for-electrophiles – bon Feb 16 '16 at 18:55
2 Answers
In the presence of these strong acids the $\ce{-NMe2}$ group is protonated, and the protonated form is electron-withdrawing via the inductive effect. This discourages attack at the electron-poor ortho position.
Under the conditions I know for that experiment, you get a mixture of para- and meta-product, but no ortho-product due to steric hindrance.
- 6,123
- 2
- 37
- 61
In these acidic conditions, the lone pair on the nitrogen will abstract a proton of the acid in an acid-base fashion. This protonated amine will then be considered a meta-directing group. It is a deactivator that will draw electron density away from the ring (because nitrogen, a relatively electronegative atom, will have a positive charge).
The best way to get around this is to N-acylate. This can be done by first reacting the mono-substituted amine with acetic chloride ($\ce{CH3COCl}$) with pyridine to get an amide. That way, the ring is still activated and the lone pair on the amide nitrogen will not interfere with acids. It will direct ortho-para (para favored due to sterics).
- 965
- 1
- 10
- 10