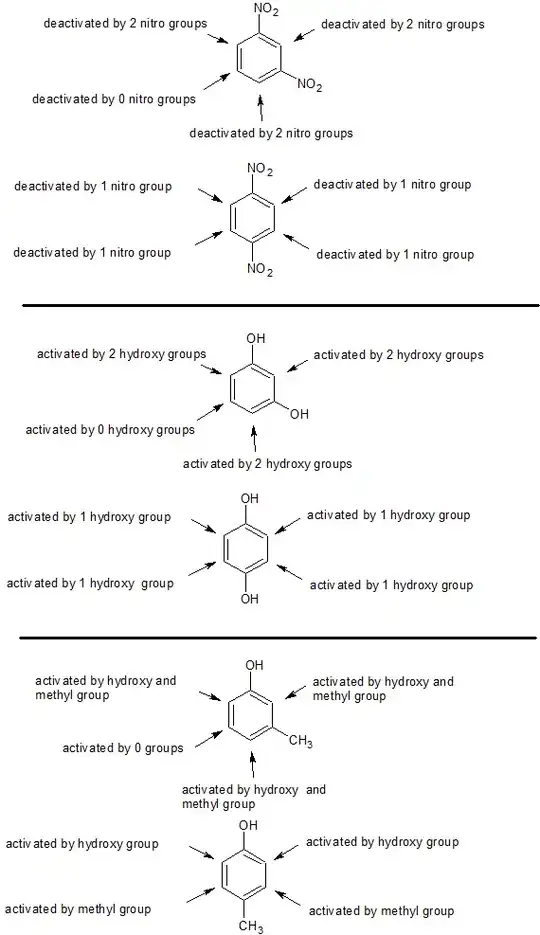
In the following pairs of molecules, which is more reactive towards electrophilic substitution reaction?
1) 1,4-dinitrobenzene or 1,3-dinitrobenzene (don't consider the ortho isomer)
2) benzene-1,3-diol or benzene-1,4-diol (don't consider the ortho isomer)
3) 4-methylphenol or 3-methylphenol
4) 4-nitrotoluene or 3-nitrotoluene
I am having a conflict of concept.
In the first pair of molecules, according to resonance the presence of one nitro will make C3 negative and C4 positive. So if another nitro comes at C4 then the positive charge at C4 will intensify as nitro draws more electron density. If the second nitro comes at C3 then the negative charge at C3 will be absorbed by nitro. So which of these will reduce the electron density on benzene more.
In my opinion 1,3-dinitrobenzene will have less electron density on the benzene ring as (C3 is already negative due to nitro at C1) much of the electron density at C3 is absorbed. But in 1,4-dinitrobenzene the second nitro group is absorbing electron density from C4 (in which electron density is very less already due to presence of nitro at C1). So since the electron density is less at C4 then it won't be able to take much electron from ring (as its already positive and deficient of electron density).
If my concept is wrong with respect to above context, please correct me.
All of the remaining isomer pairs have same problem, but each is a bit different. I would like an explanation for each answer and especially the 1st one in detail. And try not to provide an answer by directly copying from a source (especially large) thanks.