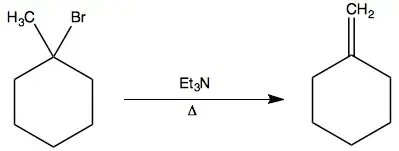
ATTEMPT:
Since $\ce{Et3N}$ is a weak base and the carbon to which $\ce{Br}$ is attached is tertiary, we can rule out $\mathrm{E2}$ and $\mathrm{S_N2}.$ As we are providing heat, the reaction is likely to go via $\mathrm{E1}$ forming a stable tertiary carbocation.
Now as the base is bulky it will knock out the most easily available $\ce{H}$, giving us the less substituted alkene.
However my text suggests that this reaction proceed via $\mathrm{E2}$ with the base taking a $\ce{H+}$ and knocking off $\mathrm{Br}$ to give the same final product.
In general with amines out of $\mathrm{E1}$ and $\mathrm{E2}$ which reaction is preferred if there is a possibility of stable carbocation formation?