Already I know that hydrogen, all the halogens, nitrogen and oxygen forms diatomic molecules. But I am confused about $\ce{Na}$? So I would like to know about that.
-
I haven't seen any cases approving $\ce{Na2}$'s existence, but $\ce{Li2}$ exists for sure. http://en.wikipedia.org/wiki/Dilithium – M.A.R. Jun 10 '15 at 08:16
-
1The molecule $\ce{Na2}$ is well known in the gas phase (and $\ce{ Li2, K2}$), and has been studied since approx 1929! The ground state has a (long) bond length of $0.3078$ nm, vibrational frequency of $159 \pu{cm^{-1}}$ and rotational constant $0.1547 \pu{cm^{-1}}$. At least four excited state are known. – porphyrin Feb 09 '17 at 16:14
2 Answers
According to molecular orbital theory, disodium should be stable in the gas phase, with a bond order of one. The molecular orbital diagram is the same for all the alkali metals since they all have one valence electron in an $s$ orbital.
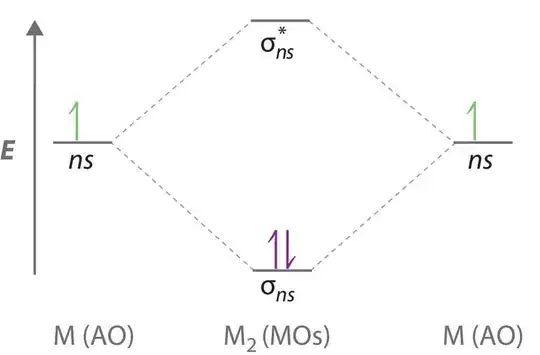
NIST chemistry webbook has a small page on disodium and quotes an enthalpy change of formation of $142.07~\mathrm{kJ~mol^{-1}}$ which is moderately endothermic.
This page has a brief discussion of alkali metal dimers and claims that they are all stable in the gas phase, in agreement with the prediction from MO theory.
- 15,369
- 13
- 62
- 91
-
And are there any elements behave like this except what I have mentioned above and Na? – On the way to success Jun 10 '15 at 15:17
-
The quoted enthalpy of formation, +142 kJ/mol Na2, is not the whole story. It should be compared with the atomization energy given by Chemistry Libretexts as +107 kJ/mol Na atoms. Thus sodium atoms in the gas phase pair up with a bond enthalpy of -72 kJ/mol Na2. – Oscar Lanzi Aug 04 '21 at 11:33
The disodium molecule $\ce{Na2}$ has first been observed by M. Polanyi and collaborators in the diluted flame of sodium vapor and chlorine $\ce{Cl2}$. When sodium metal is heated in a vacuum, it gets vaporized. If now a tiny amount of gas $\ce{Cl2}$ is sent into this vapor, the famous yellow flame of $\ce{Na}$ is produced at the point where both gases are in contact to one another. This diffusion flame is due to three consecutive reactions. First : $$\ce{Na + Cl2 -> NaCl + Cl}$$ Then the chlorine atom cannot react with atomic sodium $\ce{Na}$ since there is no third body to remove the reaction energy. So the $\ce{Cl}$ atom does react with the dimer $\ce{Na2}$ in the reaction $$\ce{Cl + Na2 -> NaCl + Na'}$$ and this reaction is exothermic enough to produce an excited $\ce{Na'}$ atom. Sorry ! this $\ce{Na'}$ should have been printed as $\ce{Na}$ with a star, but my keyboard refuses to print this symbol (*) in index. Now this excitation energy ir reemitted as the yellow D-line of sodium ($\pu{589.89 nm}$) $$\ce{Na' -> Na + h\nu }$$ This was the first proof that the dimer $\ce{Na2}$ exists in the sodium vapor.
Ref,: 1) M. Polanyi, Atomic reactions, William and Norgate, London, 1932. 2) M. G. Evans, M. Polanyi, Transactions Faraday Soc. 35, 178, 1935
- 28,241
- 3
- 29
- 61