Whether be it from a textbook or teacher, precipitation reactions always occur by rearranging the elements:
For example
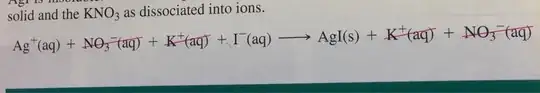
But then, aren't there other factors you have to bare in mind when predicting such reactions? Like electronegativity: obviously the potassium ion is much more electronegative than Silver ion, so it wouldn't be that easy for potassium to split up with iodine.