How can we find the bond angle between 3 atoms in a compound? Please specify an equation which can be used for all compounds.
Asked
Active
Viewed 2,137 times
1
ron
- 84,691
- 13
- 231
- 320
satwik satapathy
- 31
- 3
-
There is no such equation. The bond angle can be estimated by some simple rules, such as VSEPR theory. – Geoff Hutchison Nov 25 '14 at 14:45
-
If you know atomic coordinates (say, from a single-crystal diffraction experiment), then the task is basically the same as finding an angle between two vectors in 3D space. – andselisk Oct 30 '19 at 10:49
1 Answers
8
In carbon compounds Coulson's Theorem can be used to relate bond angles to the hybridization indices of the bonds involved.
$$1+\lambda_{i} \lambda_{j} \cos(\theta_{ij})=0$$
where $\ce{\lambda_{i}}$ represents the hybridization index of the $\ce{C-i}$ bond (the hybridization index is the square root of the bond hybridization) and $\ce{\theta_{ij}}$ represents the $\ce{i-C-j}$ bond angle.
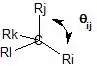
Let's look at some examples.
- In the case of methane, each $\ce{C-H}$ bond is $\ce{sp^3}$ hybridized and the hybridization index of each $\ce{C-H}$ bond is $\sqrt3$. Using Coulson's theorem we find that the $\ce{H-C-H}$ bond angle is 109.5 degrees.
- In the case of the methyl carbocation ($\ce{CH3^{+}}$) the $\ce{C-H}$ bonds are $\ce{sp^2}$ hybridized and the hybridization index of each $\ce{C-H}$ bond is $\sqrt2$. In this case, using Coulson's theorem we find that the $\ce{H-C-H}$ bond angle is 120 degrees.
- Finally, in the case of acetylene the $\ce{C-H}$ bond is $\ce{sp}$ hybridized and the hybridization index of each $\ce{C-H}$ bond is $\sqrt1$. In this case, using Coulson's theorem we find that the $\ce{C-C-H}$ bond angle is 180 degrees.
Conversely, if the bond angle is known, the hybridization can be determined.
Martin - マーチン
- 44,013
- 13
- 159
- 319
ron
- 84,691
- 13
- 231
- 320