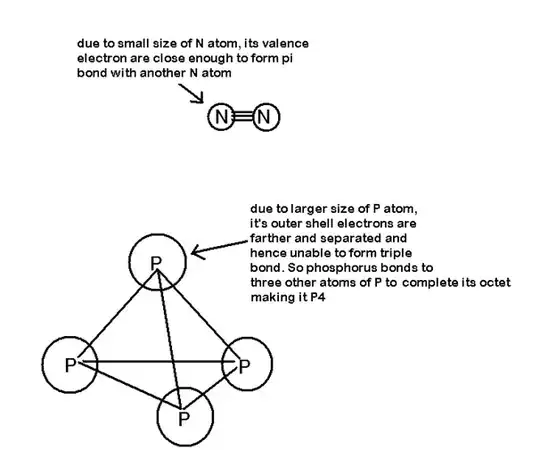
$\ce{N2}$ is a gas
$\ce{P4}$ is a solid
$\ce{N}$ and $\ce{P}$ are in the same group in the periodic table
The bond enthalpies are:
$\ce{N#N}$ triple bond is $949~ \mathrm{kJ/mol}$
$\ce{P#P}$ triple bond is $490~ \mathrm{kJ/mol}$
$\ce{P-P}$ single bond is $200 ~ \mathrm{kJ/mol}$
$\ce{N-N}$ single bond is $159-296 ~ \mathrm{kJ/mol}$
So why does $\ce{P}$ go to $\ce{P4}$ not $\ce{P2}$ and why does $\ce{N}$ go to $\ce{N2}$ and not $\ce{N4}$?