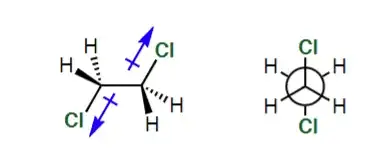
I understand why the dipoles would align in a polar solvent to maximise dipole-dipole interactions but wouldn't the replulsion due to like charges destabilise the molecule? Is it a MO effect instead? An undergraduate level response would be appreciated.
-
Suppose you just have H-Cl molecule, can you draw the dipole moment directions? – AChem Sep 22 '23 at 12:26
-
Yes, they're aligned, in the same direction. – betatester13 Sep 22 '23 at 12:34
-
No, what is the direction for H-Cl's dipole moment? There is only one dipole moment so the question of alignment does not arise. Just think about H-Cl. What is the direction of dipole moment arrow? It is a vector, right? – AChem Sep 22 '23 at 12:50
-
Yes it starts at H and ends at Cl – betatester13 Sep 22 '23 at 14:22
1 Answers
When dipoles are free to move, all else being equal, they will tend to align "tail-to-tip". That is the case, for example, when the dipoles belong to different molecules in a liquid. But the two polarized bonds within a single molecule of dichloroethane are not free to move to arbitrary positions. The two carbons are stuck together by a bond, and the only degree of freedom is rotation around that central carbon-carbon bond.
This rotation doesn't change the distance between the two positive carbon atoms. It also doesn't change the distance between either carbon atom and either chlorine atom. So the only relevant effect of this rotation is to change the distance between the two negatively charged chlorine atoms.
Therefore, the most stable conformation is the one where the negatively charged chlorines are furthest apart, since that minimizes the repulsive force.
- 838
- 1
- 9