You want to blend $\ce{C}$, $\ce{Li}$ and $\ce{F}$ into one molecule and state "found" $\ce{CF5Li}$ as a solution. From this I take, there is no constraint on the numbers of atoms as long each element is present by one atom, or multiple.
With 3,3,3-trifluoropropyne there is an entry on PubChem beyond of living in silico only, with a CAS registry number 661-54-1 (reference). The compound is literature known since 1950,(1) described with as "a gas of boiling point c $\pu{-48^\circ{}}$ with an odour reminiscent of that of acetylene". Though rather on the expensive side for such a small molecule ($\ce{C3HF3}$, $M = \pu{94.04 g/mol}$), it is commercially available; for \$US 1,010.00 MilliporeSigma sells you $\pu{10 g}$ in a cylinder.
Attempting chemical overkill on paper, one could deprotonate this ethyne with butyl lithium (BuLi), formally creating $\ce{C3F3Li}$:
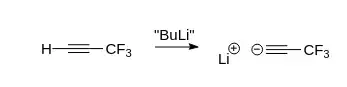
From a practical perspective, however:
the reaction has to be carried out at a temperature of $\pu{-48 ^\circ{}C}$, or below, to retain 3,3,3-trifluoropropyne in the liquid phase. Likely, a reaction temperature considerably lower will better dissipate the heat of reaction.
butyllithium is a base way stronger, than typically required to abstract a protons attached to an ethyne. With the presence of a conjugated, electron withdrawing trifluoromethyl group ($\ce{-CF3}$), sodium hydride often used for such a reaction equally would be multiple orders of magnitude stronger, than necessary.
The byproduct of butyl lithium's reaction as a base is butane. Wikipedia's corresponding property box lists a boiling point of pure $n$-butane at atmospheric pressure in the range of $\pu{-1 \ldots +1 ^\circ{}C}$, so this doesn't leave the reaction mixture. This possibly could hamper attempts to isolate the envisioned product, $\ce{CF3C#CLi}$.
Haszeldine already described the preparation of compounds where hydrogen was replaced by a metal, $\ce{CF3C#CCu}$, $\ce{CF3C#CAg}$, and $\ce{(CF3C#C)2Hg}$ with copper, silver and mercury where "the first acetylides decompose quitely on gentle heating and violently when heated rapidly." Maybe $\ce{CF3C#CLi}$ is similar, i.e. unstable. Given the formation of e.g., tetramers for butylithium, the notation of $\ce{CF3C#CLi}$ as an isolated molecule might not be the correct representation of the structure of the reaction product, either.
This doesn't sound like an easy synthesis.
Because you mentioned your background leans more toward computation, than chemistry: the systematic generation of molecules in silico stays a field of ongoing research. Challenges include the identification of efficient algorithms (and their implementation) to shuffle the atoms to build chains, and rings. Then you need quality checks to retain molecules which are chemically plausible e.g., by count of valences (how many bonds an atom typically engages), or ring strain. Last but not least, you equally want to screen these structures for useful chemical and physical properties to identify interesting candidates for drug design, or material science -- still before performing any chemical synthesis in the lab.
Thus, examples like MAYGEN and OMG, or Reymond's massive compilations GBD-11, GDB-13, and GDB-17 can only provide a glimpse.
addition past acceptance of the answer: User @Mithoron suggests in a comment pentafluorobenzene as an alternative, i.e. implies a reaction like

This would be much more affordable ($\ce{C6HF5}$, $M = \pu{168.06 g/mol}$) per gram, as well per mole as the same source as above
currently sells $\pu{5 g}$ for \$US 40.00. In addition, the analogue phenyl lithium (lacking the fluorine atoms) is readily available and used a chemical reagent solution. Thus, the synthesis departing from pentafluorobenzene likely is less troublesome than the one on 3,3,3-trifluoropropyne, too.
(1) Haszeldine, R. N. Synthesis of 1:1:1 Trifluoropropyne. Nature 1950, 165, 152–153; doi 10.1038/165152b0
(2) Peironcely, J.E., Rojas-Chertó, M., Fichera, D. et al. OMG: Open Molecule Generator. J Cheminform 2012, 4, 21; doi 10.1186/1758-2946-4-21 (open access); http://sourceforge.net/p/openmg
(3) Yirik, M.A., Sorokina, M. & Steinbeck, C. MAYGEN: an open-source chemical structure generator for constitutional isomers based on the orderly generation principle. J Cheminform 2021, 13, 48; doi 10.1186/s13321-021-00529-9 (open access); https://github.com/MehmetAzizYirik/MAYGEN
(4) https://www.gdb.unibe.ch/downloads/
(5) What are the GDB-13 criteria for "synthetically accessible organic molecules"?

