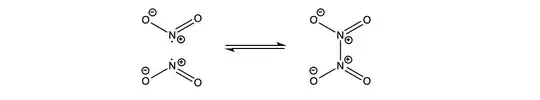
Which is the correct structure of $\ce{NO2}$? While searching the internet I found out that
$\ce{NO2}$ have a coordinate and two covalent bonds. $\ce{N}$ will have a positive charge. $\ce{O}$ (coordinate bond) will have a negative charge (this is the part which I don't understand. As $\ce{O}$'s octet is complete, why will it have a negative charge?)
$\ce{NO2}$ came from $\ce{HNO2}$ that's from $\ce{O}$ have a negative charge. but in this molecule, there is no $\ce{O}$ with coordinate bond.
In one molecule $\ce{N}$ have one electron and positive charge and in other two electrons and no charge?