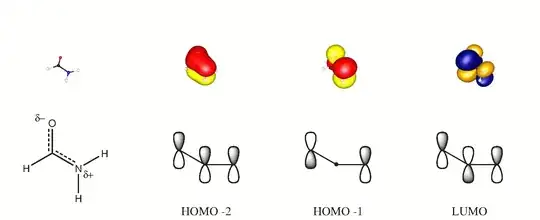
In formamide, the nitrogens appear to be $\ce{sp^3}$ hybridized, implying tetrahedral geometry. However, analysis shows that the molecule is actually very nearly planar with bond angles close to 120 degrees.
EDIT: as suggested by Martin and another poster, hybridization is a rough concept. So perhaps the hybridization of nitrogen upon further analysis should best be described as somewhere inbetween $\ce{sp^3}$ and $\ce{sp^2}$. This however would still necessitate planarity, correct? Pi bonds are formed through the above and below electron-pairing in p-orbitals; effective bonding is achieved when these p-orbitals are parallel with respect to each other.
I'm thinking this has to do with the partial double bond character in the molecule (also appears to be some ionic character to the molecule - likely due to electron-withdrawing effects of the nitrogen and the oxygen).

This is the standard answer. However, would intramolecular hydrogen bonding also play a role? Couldn't there be a hydrogen bond between the peripheral hydrogen on the nitrogen and the oxygen? Couldn't this also help with achieving the 120 degree bond angles?