I built a calculator to calculate a planet's atmospheric pressure at an altitude with the formula below.
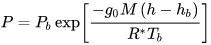
How does greenhouse effect relate to the formula above? How to calculate it?
I compared the results with the in-game data including same input data but it becomes less accurate the farther it is from sea level so something might be missing behind the scenes.
Heres the ingame data:
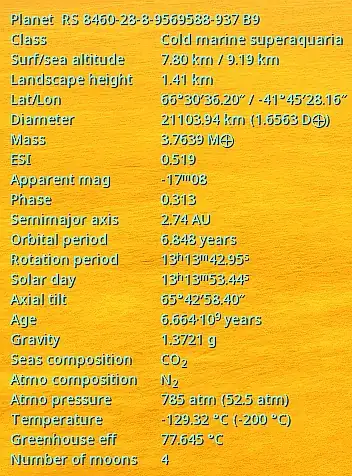
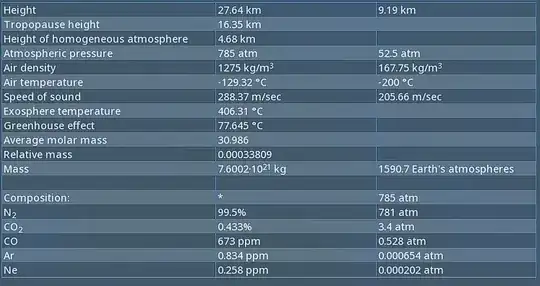
And my calculator results:


As you can see, the atmos pressure in the in-game data and calculator are not reasonably close enough, maybe I could accept at least around ±1
I searched and asked some people about greenhouse effect on atmospheric pressure
Greenhouse effect isn't directly related to pressure. It's indirectly related to the partial pressure of greenhouse gases
The most direct thing you could relate it to is the optical depth of the atmosphere in infrared (going as optical depth to the 1/4th power), but calculating optical depth from composition and pressures isn't straightforward either.
At the most basic and as a learning tool, they're often treated as a surface + single layer with incoming radiation acting independently of re-emitted radiation. For more complexity, have multiple layers. For more complexity, treat each volume element and its emission in terms of solid angle and each tiny bit of wavelength.
Apparently, the game was more of an emulator rather than a simulator, it could just make up data to make it seem believable and consistent with findings instead of full calculations.
Other possible factors could be lapse rate (the formula above is the version that implies that lapse rate is 0) or Stefan-Boltzmann's Law/black-body radiation/effective temperature.
How about lapse rate, is it involved?
– bridgeofdata Jan 31 '23 at 02:19Why is vibrational degree of freedom counted in N value for CO₂ but not like other N₂, O₂ and other similar molecules? And how did they get γ = 1.3 with 7, I calculated that it has to be around 9.0-9.1. In the first place, its my first time learning about degrees of freedom. I also just figured out what linear molecule is. – bridgeofdata Feb 01 '23 at 15:01
From my questions & answers, you definitely know that I don't have anything in physics except the basics. – bridgeofdata Feb 02 '23 at 13:19
Does 1/m^3 for abundance mean 1 atom or mol per cubic metre?
– bridgeofdata Feb 03 '23 at 09:53Edit: I got some progress on calculating abundances, now only the mean opacity left
– bridgeofdata Feb 04 '23 at 16:25